Nano Letters, the academic journal in nano science under American Chemical Society, published “The Electrochemistry with Lithium versus Sodium of Selenium Confined To Slit Micropores in Carbon” online, a study conducted by Xin Sen, a young fellow from School of Chemistry and Chemical Engineering, HFUT.
The study was done in collaboration with research groups of Institute of Chemistry, Chinese Academy of Sciences (CAS), Materials Science and Engineering Program and Texas Materials Institute, the University of Texas at Austin, and Department of Materials Science and Engineering and Department of Chemistry, Stanford University.
Through precise modulation of nanochannels, researchers successfully fabricated a new-type selenium anode material based on microporous carbon and carried out a systematic study of its electrochemistry in high power density lithium/sodium-selenium rechargeable battery.
Group VIA, including oxygen, sulfur, selenium, Tellurium and Polonium, and its chemical compounds, are highly applicable to making high power density Lithium/Sodium-Selenium rechargeable batteries for its cheap cost and abundant source. The research group has conducted a series of studies in this field. In studying the selenium anode, the group found that its volumetric capacity (3250 mA h cm −3. ) comparable to that of sulfur anode (3470 mA h cm-3). Meanwhile, its electrical conductivity is far better than that of sulfur, thus having great electrochemical reactivity with lithium ion and sodium ion. Thus, the alkali metal-selenium rechargeable batteries would be a very competitive product in the market of high-end electronic products.
Substitution of selenium for sulfur in the cathode of a rechargeable battery containing Sx molecules in microporous slits in carbon allows a better characterization of the electrochemical reactions that occur. Paired with a metallic lithium anode, the Sex chains are converted to Li2Se in a single-step reaction. With a sodium anode, a sequential chemical reaction is characterized by a continuous chain shortening of Sex upon initial discharge before completing the reduction to Na2Se; on charge, the reconstituted Sex molecules retain a smaller x value than the original Sex chain molecule. In both cases, the Se molecules remain almost completely confined to the micropore slits to give a long cycle life.
Xin Sen, the first author of the paper, has already published 30 plus papers on famous international journals. His studies have been supported by the Young Scientists Fund of the National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities of Ministry of Education of China, CAS and the US Department of Energy.
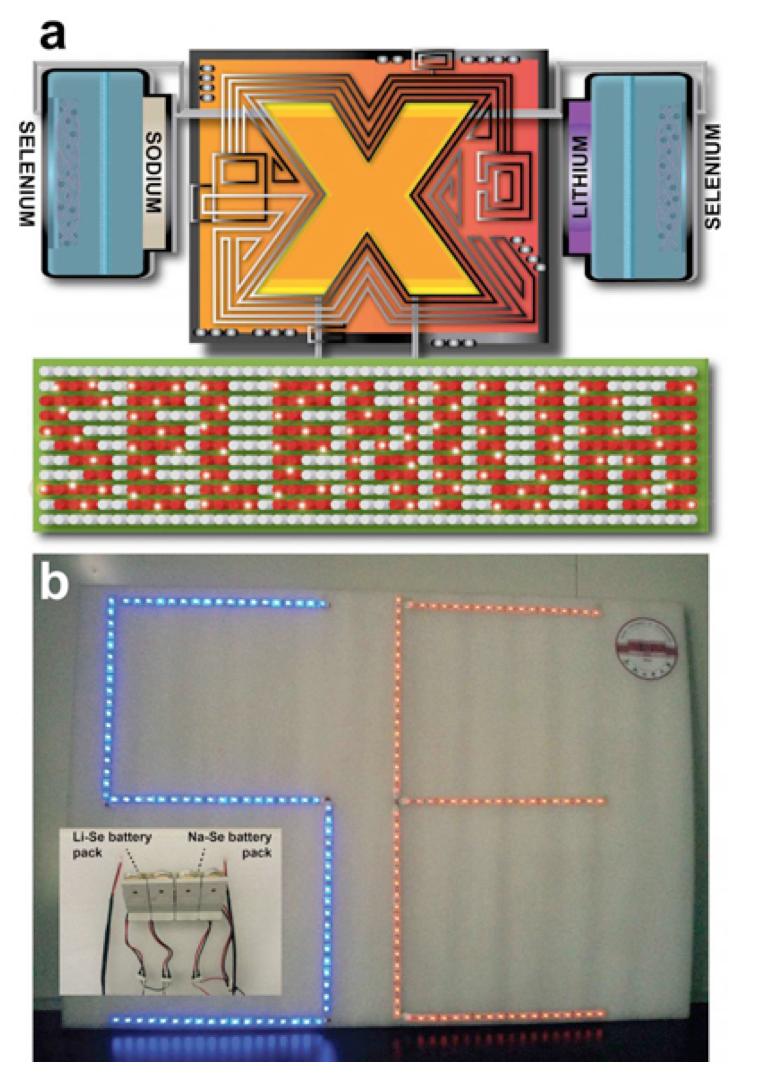
Figure A: application of lithium/sodium-selenium rechargeable battery in electronic products
Figure B: LED panel driven by lithium/sodium-selenium battery sets
Written by & photo credit: Xin Sen
Edited by: Zhou Hui
编辑:闵璇


 HOME
/
Content
HOME
/
Content
